MnBi2 is a permanent magnet
Creating and understanding new permanent magnets requires an understanding effect of orbital angular momentum on coercivity. Permanent magnets are at the core of technologies ranging from energy generators to energy storage. The next generation of magnetic technology not only needs discovering new magnetic materials but also being able to tune the magnetic properties. Pressure enables tuning inter-atomic spacing and therefore their interactions much better than any other variable such as temperature or composition. Synchrotron based, in-situ measurements of structure and magnetic properties present a unique opportunity to synthesize and characterize new magnetic materials.
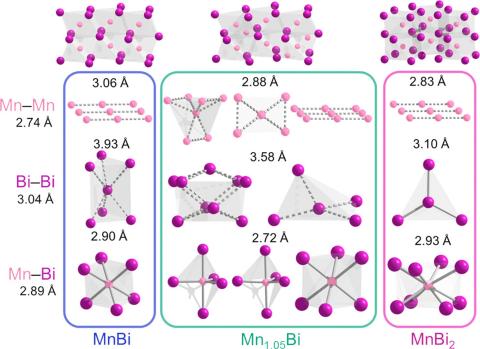
One approach to understanding the interplay of orbital angular momentum and coercivity is achieved by incorporating high Z elements into binary compounds to maximize the spin-orbit coupling. Mn-Bi binary system is one such system which already contains MnBi, a permanent magnet with a large coercive field. MnBi2 is a new compound that can be synthesized at high pressure (8.3 GPa, above 400 K) but cannot be recovered on decompression necessitating measurements need to be performed at high pressures in a diamond anvil cell (DAC). Magnetic measurements in a DAC are difficult due to the high shielding effects of the cell assembly needing in-situ techniques such as neutron diffraction, NV centers mediated flux imaging, AC susceptibility or x-ray circular dichroism spectroscopy (XMCD). Of these, XMCD is a powerful technique that can be tuned to specific core-level electronic transitions that can therefore elucidate element-specific contributions to the overall magnetic behavior.
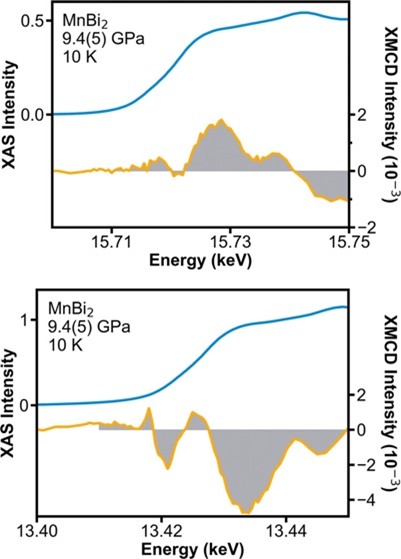
This work by Catherine Badding and co-authors was recently published in J. Am. Chem. Soc. (147, 25129-25135 (2025)).