Low-density liquid water upon rapid decompression
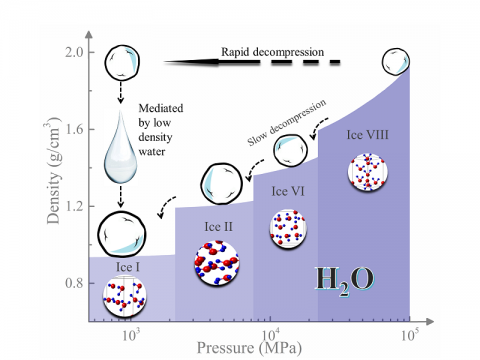
At temperatures (<319 K or <46 oC) where life is sustained, water is an abnormal liquid, having a number of anomalous properties. For instance, water displays minima of isobaric heat capacity at 308 K and isothermal compressibility at 319 K which are related to entropy and density fluctuations, respectively. It has been widely accepted that water’s anomalies are not a result of simple thermal fluctuation, but are connected to the formation of various structural aggregates in the hydrogen bonding network. To understand water’s anomalous behavior, a two-liquid model with a high-density liquid (HDL) and a low-density liquid (LDL) has been proposed from theoretical simulations. However, it has been experimentally challenging to probe the region of the phase diagram of H2O, often referred to as water’s no man’s land, where the LDL phase is expected to occur. A team at HPCAT overcame the experimental challenge by adopting a new kinetic pathway to access the region of LDL via decompression of a high-pressure crystal. In contrast to the liquid water, the LDL is found to have the tetrahedral network fully developed. The relationship of LDA, LDL, and supercooled liquid can be used to explain water’s anomalous properties. At high temperatures above ∼319 K, water exhibits normal liquid behavior (dominated by HDL), in that both heat capacity and compressibility increase as it is heated. As water is cooled from 319 K down to supercooled temperatures, the structural fluctuations between LDL and HDL may cause the anomalous properties of water. The anomalous region with structural fluctuations may extend down to around the Widom line. At temperatures far below the Widom line, water may exhibit normal liquid again dominated by the fully developed tetrahedrally structured LDL observed in this study. More in C. Lin et al. Proc. Nat. Acad. Sci., doi.org/10.1073/pnas.1716310115, (2018)